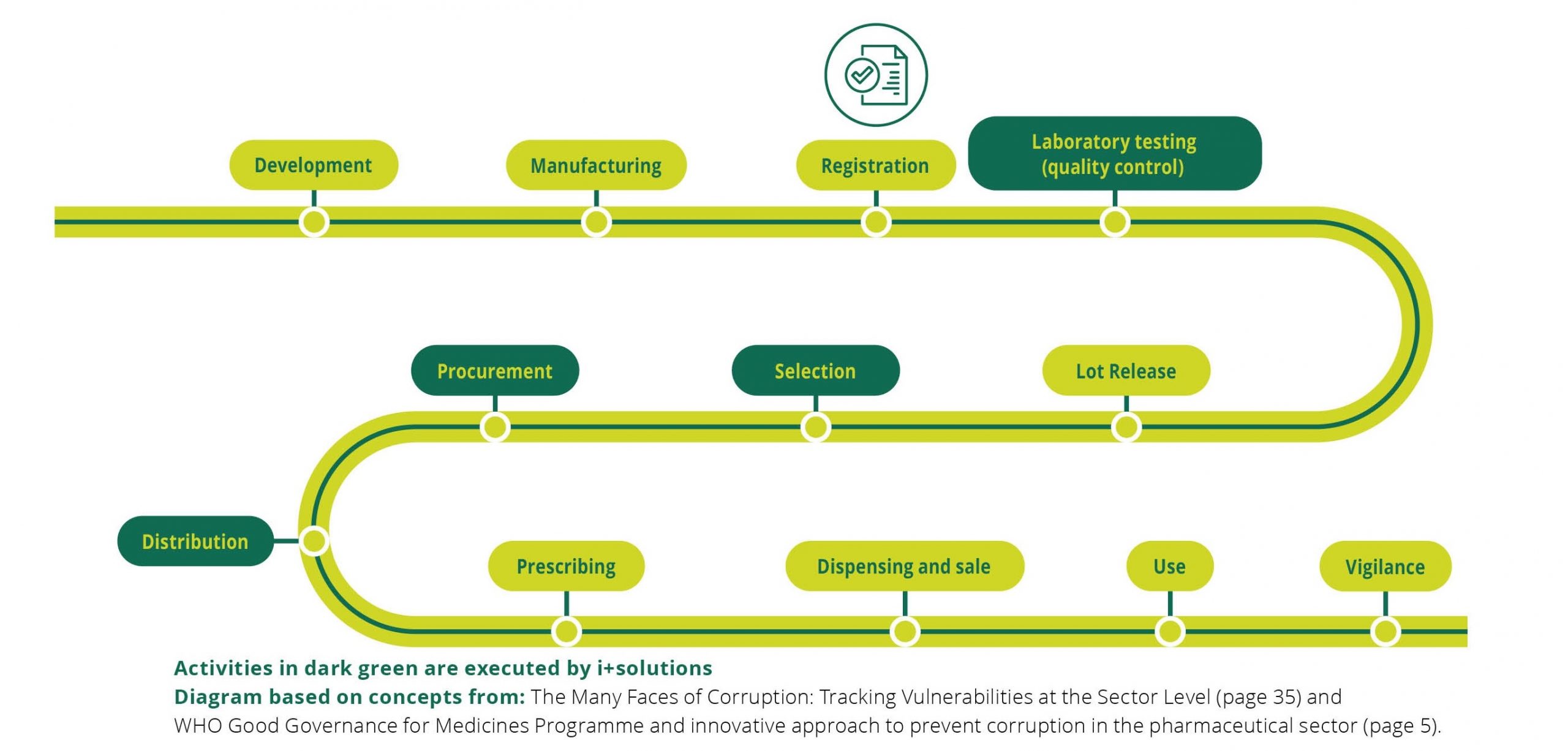
At i+solutions, quality of the medicines we procure is essential for the achievement of our ambitions. To support the objectives of our organization, we implement and maintain a Quality Management System (QMS). The QMS is based on the requirements of ISO 9001:2015, Good Distribution Practices (EU GDP and WHO GDP) and WHO’s Model Quality Assurance System For Procurement Agencies (MQAS).
The QMS is built on i+solutions’ defined processes, organization and responsibilities, in order to guide us in meeting our quality objectives. In a broader sense, it ensures that our services always meet the regulatory and customer requirements.
i+solutions’ Management, QA department and staff are responsible for quality through the QMS, seeking improvement by constant review, involving suppliers and sub-contractors.
Quality is a responsibility of every employee within the organization.
 + Quality Manual
+ Quality Manual
+ Product and vendor qualification
+ Self-inspections and audits
+ Dossier and documentation review
+ Quality Control testing
+ Handling quality incidents and CAPAs
+ Change control
+ Handling complaints and recalls
+ Handling products during storage and distribution
(Traceable in ERP system)